Introducton of Chemical reaction
Chemical reaction, is the Process in Which One or More Substances, the reactant, are Converted to One or More Different Substances, the Products. Substances are Either Chemical Elements or Compounds. A Chemical reaction Rearranges the Constituent atoms of the reactants to Create Different Substances as a Products. A Chemical reaction is a Process that leads of the Chemical reaction Transformations of a One Set of Chemical Substances to Another.

Table of Contents
ToggleWhat is a Chemical reaction
Chemical reaction
A Chemical reaction is defined as a Process by Which 2 or More Substance react With Each Other
to Form New Substance.
Chemical reaction take place when there is:
Change in State
Change in Colour
Evaluation of a Gas
Change in Temperature
What is a Chemical equation
Chemical equation is a Symbolic representation of a Chemical reaction in the Form of Symbols and Formulae Where reactants are Written on Left Hand Side with a Plus Sign Between them and Product are Written on right hand Side with a Plus Sign between them :-
For Example:- The Burning of Magnesium in air is Written as:
Magnesium + Oxygen Magnesium Oxide
(Reactants) (Product)
Mg + O2 MgO

Basic Concepts of Chemical reaction
A Chemical reaction is When two or more Molecules Mix together to Make Something New. The things that Mix together are Called Reactants, and What they Make is Called Products.
Chemical reactions are really important in lots of things we do, like Making things in Factories, doing traditional Practices, and even in our Daily routines. They’re always happening around us, like when iron Rusts, When we Make Pottery, or When Wine Ferments.
When a Chemical reaction happens, there’s usually a Change we can see, like Something getting Hotter, a Color Changing, or Stuff Settling Out of the Mixture.
During a Chemical reaction, the atoms or tiny parts in Molecules Come together to Form new Connections, Making new Stuff Without destroying any of the atoms.
How Fast a reaction happens depends on things like how Much Pressure there is, How hot it is, and How Much Stuff is Mixed together.
Types of Chemical reaction
Decomposition reaction.
Combination reaction.
Combustion reaction.
Neutralization reaction.
Single Displacement reaction.
Double Displacement reaction.
Precipitation reaction.
Redox reaction.
What is a Decomposition reaction
A Decomposition reaction Can be defined as a Chemical reaction in Which One reactant Breaks down into two or more Products. Any Reactant Substance Decomposed by the Action of Heat or by the Application of Electricity.

What is a Combination reaction
Composition reaction Produces a Single Substance from Multiple Reactants. Combustion reactions are the Combination of Some Compound with Oxygen to Make Oxides of the other Elements as Products (although Oxygen atoms react to make O2).

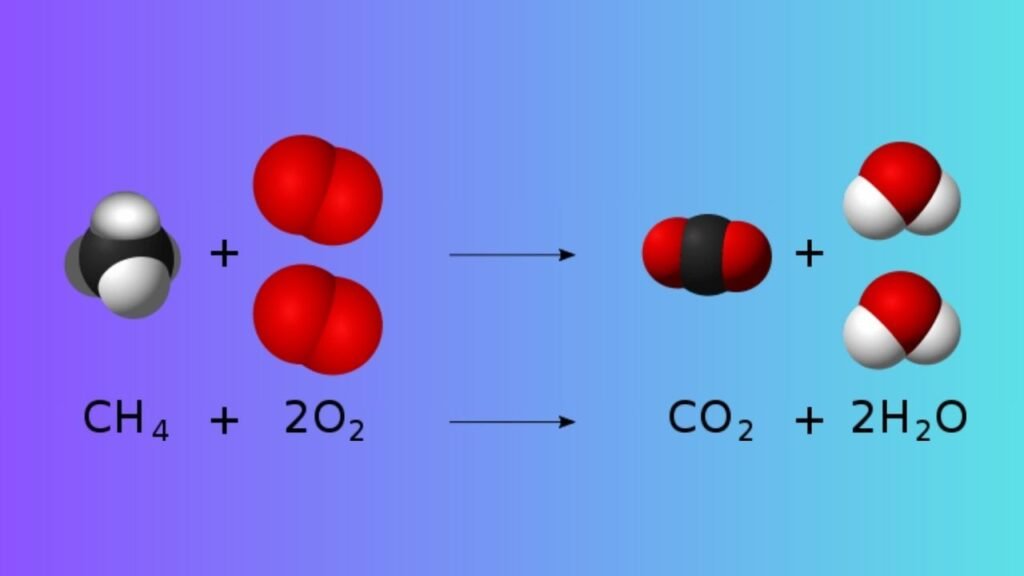
What is a Combustion reaction
Combustion reactions are generally Highly Exothermic Redox reactions Between an Oxidant and a Fuel. The Product Formed in a Combustion reaction is Usually the Oxidised Fuel (which is mostly liberated in the gaseous state). This is Often Referred to as Smoke.
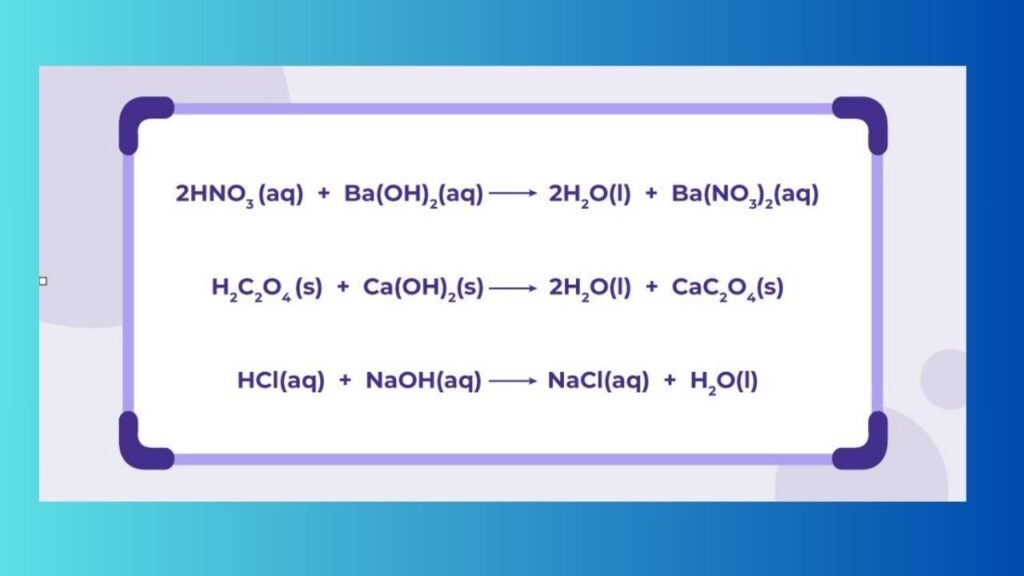
What is a Neutralization reaction
A Neutralization reaction is When an Acid and a Base react to Form Water and a Salt and Involves the Combination of H+ ions and OH- ions to Generate Water. The Neutralization of a Strong Acid and Strong Base has a PH Equal to 7.
What is a Single displacement reaction
A Single replacement reaction, Sometimes Called a Single Displacement reaction, is a Reaction in Which one Element is Substituted for Another Element in a Compound. The Starting Materials are always Pure Elements, Such as a Pure Zinc Metal or Hydrogen Gas, Plus an Aqueous Compound.

What is a Double displacement reaction
Double displacement reactions are those in Which two Chemical Substances react by Exchanging ions to Produce two New Molecules. Positive ions Exchange Negative ions in the Double Displacement Process. Ionic Chemicals dissolved in Water Undergo a lot of Double Displacement Processes.

What is a Precipitation reaction
The Terms of ‘Precipitation reaction’ Can be defined as “ a Chemical reaction Occurring in an Aqueous Solution Where two ionic bonds Combine, Resulting in the Formation of an Insoluble Salt”. These Insoluble Salts Formed in Precipitation reactions are Called Precipitates.

What is a Redox reaction
An Oxidation-reduction reaction is any Chemical reaction in Which the Oxidation Number of a Molecule, atom, or ion Changes by Gaining or Losing an Electron. Redox reactions are Common and Vital to Some of the Basic Functions of Life, Including Photosynthesis, Respiration, Combustion, and Corrosion or Rusting.

Equation of the Chemical reaction
Chemical reactants are Converted to Products, and the Process is Symbolized by a Chemical equation. For example, Iron (Fe) and Sulfur (S) Combine to Form Iron Sulfide (FeS). Fe(s) + S(s) → FeS(s) The Plus Sign indicates that Iron reacts with Sulfur.


Important Points to Remember in Chemical reaction
In a Chemical changes, a different Substance is Made, While in a Physical change, the Substance just Changes how it looks or feels.
The things that Mix together to Make Something new in a Chemical reaction are called reactants, and what they make is called products.
Chemical reactions obey the rule that Matter is Conserved. This Means that no Matter is lost or gained during the reaction, only Rearranged to form the New product.
See also
Thanks for Visiting. Have a Good Day
Visit my Website for Amazing Blogs
This Content is Amazing

Resources of Weather || Defination || Types || Benifits || Easily Explanation
March 30, 2024
No Comments
Read More »

Resources of Water || Defination || Types || Benifits || Easily Explanation
March 24, 2024
No Comments
Read More »

Properties of Soil || Types || Conversion || Structure || Easily Explanation
February 28, 2024
No Comments
Read More »


Metals and Nonmetals || Defination || Types || Uses || Easily explanation
February 22, 2024
No Comments
Read More »

Natural Phenomena || Defination || Types || Causes || Easily Explanation
February 17, 2024
No Comments
Read More »
Web Stories
प्रकृति में शुद्ध पानी के कितने स्रोत है हमें पिने के लिए शुद्ध पानी कहा से मिलता है आइये जानते है
By sciencestudy.fun
क्या आप जानते है की प्रकृति में मिट्ठी का क्या महत्व होता है मिटटी की संरचना कैसी होती है कैसे पेड़ और पौधे मिटटी से अपना खाना बनाते है आइये जानते है
By sciencestudy.fun
क्या आप जानते है बारिश के बाद ही आकाश में इन्द्रधनुष कैसे बनता है क्या विज्ञानं है इसके पीछे
By sciencestudy.fun





